Electrochemical impedance spectroscopy (EIS) is a powerful technique for studying electrochemical systems such as electrochemical cells, batteries, fuel cells, corrosion protection setups, and sensors. By differentiating processes such as charge transfer across the electrode interface, diffusion, double-layer behavior, etc., by applying small sinusoidal signals generated in random magnitudes over a wide frequency range, we invoke responses from such mechanisms. Equivalent circuits in the traditional sense can conveniently give impedance data representations; however, they do not suffice when overlapping or nonideal processes come into play. Modern physics-based modeling approaches enable the researcher to consider adsorption, mass transport, and electrode surface effects far beyond simple resistor–capacitor analogies.
EIS Real-Life Applications
Sensitivity renders EIS paramount for:
Batteries: Detects ion and electron transport at early stages of degradation and capacity fading.
Corrosion: Detects subtle interface changes between metal and electrolyte in pipelines, concrete, and marine structures.
Fuel Cells: Performance and durability improvements by separating contributions of catalyst layers, membranes, and reactant flows.
Sensors: Evaluates electrode interactions with target molecules, enabling applications like glucose monitoring.
The Limitations of Equivalent Circuits
For the simpler reactions, the impedance data frequently fit an elementary equivalent circuit: a resistor in series with a parallel resistor-capacitor pair. In a Nyquist plot, this will look like a neat semicircle corresponding to charge transfer resistance. However, rarely do real systems behave so nicely. Adsorption, diffusion, and electrode morphology will add time constants and overlapping processes with which the equivalent circuit cannot always keep up. Physics-based models are, therefore, chosen to solve the underlying electrochemical equations, thus providing a more accurate picture of how these processes may interrelate.
Consider the Nonidealities of EIS:
Important Factors
- Adsorption–Desorption Dynamics
Intermediates may adsorb on electrodes during electrochemical reactions. The changing surface coverage may, over time, change the impedance response. For instance, with copper deposition, a progressive increase in coverage of additives changes the spectra from two capacitive loops into one dominated by an inductive loop at low frequency. Such effects demonstrate the crucial nature of adsorption in the design of such systems.
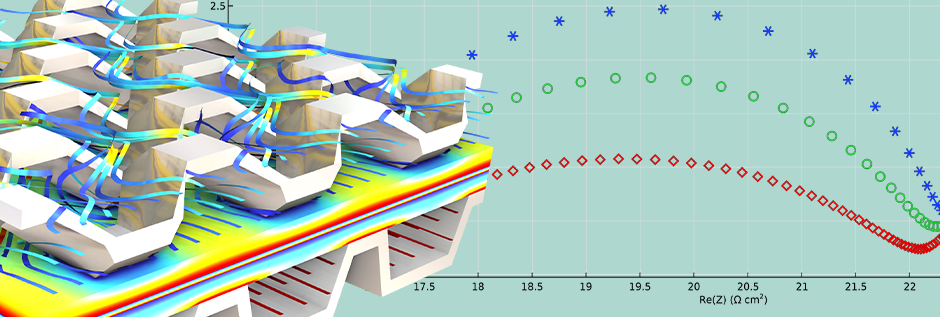
- Mass Transport Limitations
In fuel cells, the diffusion and convection of gases such as hydrogen and oxygen significantly affect performance. Through impedance plots, one can observe the changes in charge-transfer and diffusion contributions as functions of the operating potential:
- Distinct high- and low-frequency loops at intermediate voltages
- At low voltages, loops combine with overlapping time constants
- On the strongly cathodic side, diffusion is dominant, and a single huge loop appears
This sequence clearly demonstrates the ability of EIS to differentiate between reaction kinetics and transport limitations.
- Electrode Surface Effects
Surface roughness and uneven geometries alter the effective electrochemical area, thus shifting the impedance response. Accounting for electrode structures helps render better predictions in situations where morphology is important.
Handling Residual Behaviors
Sometimes, the impedance response cannot be explained by referring to adsorption, diffusion, or surface structure. A constant phase element (CPE) is then introduced to incorporate frequency-dependent effects that deviate from an ideal capacitive behavior. From a mechanistic standpoint, (CPE)behave as systems in which the mathematical expression describing a single mechanism can be modified with a continuous parameter that accounts for system complexity.
Conclusion:
Electrochemical impedance spectroscopy has remained one of the most versatile electrochemical experimental probes, and by moving beyond the simple circuit analogy to include adsorption, diffusion limitation, and surface-effects, researchers gained a more realistic view of the system behavior. Modeling platforms such as COMSOL Multiphysics support these newer approaches, albeit all electrochemical disciplines offer a general foundation.
From extending battery lifetimes to detecting early corrosion, EIS when paired with detailed physical insights continues to unlock new possibilities for innovation and reliability in electrochemical technologies.
(This article has been adapted and modified from content on COMSOL.)